
TYPE Mini Review
PUBLISHED 08 December 2022
DOI 10.3389/fspor.2022.969623
OPEN ACCESS
EDITED BY
Takeshi Hashimoto,
Ritsumeikan University, Japan
REVIEWED BY
Ming Cai,
Shanghai University of Medicine and
Health Sciences, China
*CORRESPONDENCE
Shingo Takada
Shintaro Kinugawa
SPECIALTY SECTION
This article was submitted to
Sport and Exercise Nutrition,
a section of the journal
Frontiers in Sports and Active Living
RECEIVED 15 June 2022
ACCEPTED 31 October 2022
PUBLISHED 08 December 2022
CITATION
Takada S, Fumoto Y and Kinugawa S
(2022) Ergogenic eects of caeine
are mediated by myokines.
Front. Sports Act. Living 4:969623.
doi: 10.3389/fspor.2022.969623
COPYRIGHT
© 2022 Takada, Fumoto and
Kinugawa. This is an open-access
article distributed under the terms of
the
Creative Commons Attribution
License (CC BY)
. The use, distribution
or reproduction in other forums is
permitted, provided the original
author(s) and the copyright owner(s)
are credited and that the original
publication in this journal is cited, in
accordance with accepted academic
practice. No use, distribution or
reproduction is permitted which does
not comply with these terms.
Ergogenic eects of caeine are
mediated by myokines
Shingo Takada
1,2
*
, Yoshizuki Fumoto
2
and
Shintaro Kinugawa
3,4
*
1
Department of Lifelong Sport, School of Sports Education, Hokusho University, Ebetsu, Japan,
2
Department of Molecular Biology, Hokkaido University Graduate School of Medicine, Sapporo,
Japan,
3
Department of Cardiovascular Medicine, Faculty of Medical Sciences, Kyushu University,
Fukuoka, Japan,
4
Division of Cardiovascular Medicine, Research Institute of Angiocardiology,
Faculty of Medical Sciences, Kyushu University, Fukuoka, Japan
Exercise has long been known to eectively improve and enhance skeletal
muscle function and performance. The favorable eects of exercise on
remote organs other than skeletal muscle are well known, but the underlying
mechanism has remained elusive. Recent studies have indicated that skeletal
muscle not only enables body movement, but also contributes to body
homeostasis and the systemic stress response via the expression and/or
secretion of cytokines (so-called myokines). Not only the induction of muscle
contraction itself, but also changes in intracellular calcium concentration
([Ca
2+
]i) have been suggested to be involved in myokine production and
secretion. Caeine is widely known as a Ca
2+
ionophore, which improves
skeletal muscle function and exercise performance (i.e., an “ergogenic aid”).
Interestingly, some studies reported that caeine or an increase in [Ca
2+
]i
enhances the expression and/or secretion of myokines. In this review,
we discuss the association between caeine as an ergogenic aid and
myokine regulation.
KEYWORDS
myokine, exercise mimetic, mitochondria, ergogenic aid, calcium ion (Ca)
2+
, Ca
2+
-
induced Ca
2+
release, skeletal muscle, BDNF
Introduction
Exercise training has been demonstrated to have positive effects on skeletal muscle
function and systemic exercise performance (
1, 2). The favorable effects of exercise on
remote organs other than skeletal muscle are well known (
3, 4), but the underlying
mechanism remains unclear. Recent studies have indicated that skeletal muscle not only
enables body movement, but also contributes to body homeostasis and the systemic
stress response via the secretion of soluble proteins (
3, 4). It has been suggested that
these proteins, which are cytokines and other peptides that are produced, expressed, and
secreted by muscle fibers, and exert paracrine, autocrine, or endocrine effects, should
be classified as “myokines” (
5). Not only the induction of muscle contraction itself, but
also changes in intracellular calcium concentration ([Ca
2+
]i) have been suggested to be
involved in myokine production and secretion (
6–10) (Figure 1). It is also known that
myokine secretion is promoted by the activation of 5
′
-AMP-activated protein kinase
(AMPK) signaling (
11, 12) (Figure 1).
Frontiers in Sports and Active Living 01 frontiersin.org
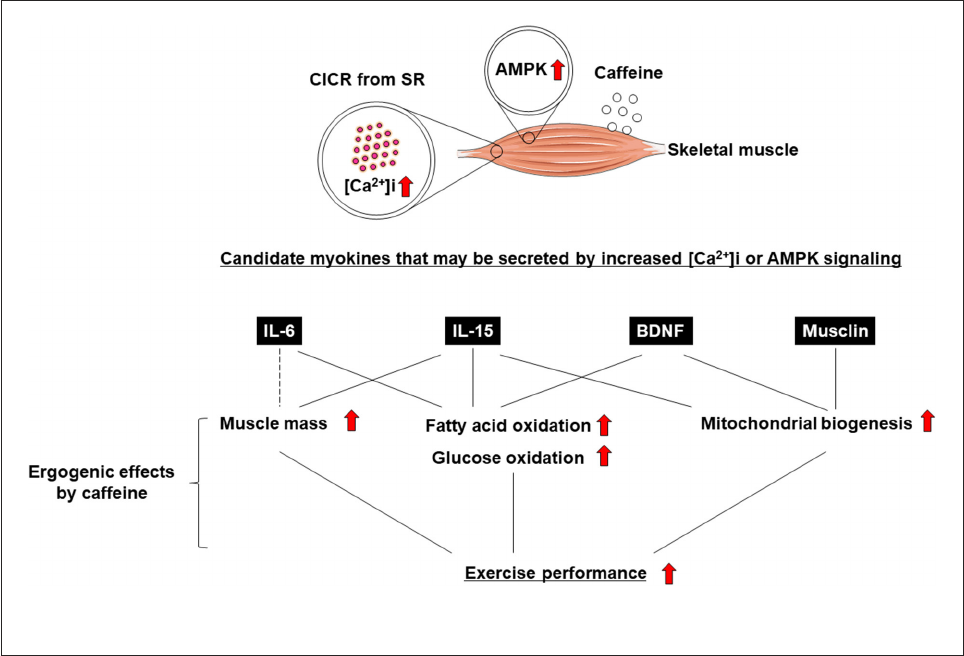
Takada et al. 10.3389/fspor.2022.969623
FIGURE 1
Ergogenic eects of caeine are mediated by myokines.
Caffeine is widely known as a Ca
2+
ionophore and/or
AMPK activator that improves skeletal muscle function
and exercise performance (i.e., an “ergogenic aid”) (
13–16)
(
Figure 1). Some studies reported that caffeine, an increase in
[Ca
2+
]i, and AMPK agonists enhance the secretion of myokines,
leading to positive effects on skeletal muscle (e.g., increased
fatty acid or glucose oxidation and mitochondrial biosynthesis)
and exercise performance. (
7–9, 11, 12, 17). In this review, we
discuss the association between caffeine as an ergogenic aid and
myokine regulation.
Evidence of the ergogenic eects of
caeine
Short-term or long-term administration of caffeine has
ergogenic effects, including enhancing fatty acid or glucose
oxidation, mitochondrial biogenesis, and muscle hypertrophy
signaling in cultured skeletal muscle cells and increasing
skeletal muscle mass in rodents (18–20). Similarly, caffeine
was identified as an ergogenic aid for exercise performance,
including aerobic endurance, mus cle strength, and muscle
endurance in humans by meta-analyses (
14). Thus, evidence
of the ergogenic effects of caffeine on exercise performance is
well-established (14, 15, 18–22).
Ergogenic eects of caeine are
mediated by myokine secretion due
to increased [Ca
2+
]i and/or AMPK
activation
One mechanism of the above-mentioned ergogenic effects
of caffeine involves calcium-induced calcium release from
the sarcoplasmic reticulum, which increases [Ca
2+
]i (
23–25).
Increased [Ca
2+
]i is involved not only in protein expression
and/or modification by enhancing the calcium signaling,
including the AMPK pathway, but also in the extracellular
secretion of proteins (
6, 7, 11, 26, 27).
Myokine production is thought to occur in response
to muscle contraction (
5, 28), but the detailed mechanism
remains unclear. Interestingly, it has been shown that myokine
secretion during acute electrical stimulation depends more on
intracellular calcium flux than on skeletal muscle contr action
itself (9). Moreover, increased [Ca
2+
]i has the potential to
promote myokine expression in skeletal muscle (
7, 8, 29). On
the other hand, caffeine is a well-known activator of AMPK
(
30, 31), and AMPK activation is involved in myokine regulation
(
11, 12). Next, we will focus on represent ative myokines
that can be regulated by increasing [Ca
2+
]i and/or AMPK
activation, which have recently been shown to be involved in
Frontiers in Sports and Active Living 02 frontiersin.org
Takada et al. 10.3389/fspor.2022.969623
the function of skeletal muscle and other organs, as well as
exercise performance.
Caeine regulates the secretion of
interleukin-6 as a myokine
Exercise was found to increase the levels of circulating
and muscle interleukin (IL)-6, which is the most well-known
myokine, in humans (
5). Similarly, caffeine was found to
increase circulating and skeletal muscle IL-6 protein levels in
mice (
17). A23187, another Ca
2+
ionophore, also increased IL-
6 mRNA expression in the skeletal muscle of mice and C2C12
myotubes (
32). Moreover, it is well known that IL-6 promotes
fatty acid and glucose oxidation in humans and in tissue culture
(
5, 33–35). Although the results are controversial, IL-6 also is
involved in muscle hypertrophy and m yogenesis (
28, 36).
In general, mM levels of caffeine have been shown to
promote Ca
2+
release from the sarcoplasmic reticulum (SR) by
acting directly on the r yanodine receptor (RyR) (
6, 37). Ducreux
et al. showed that upon activation of the RyR by the RyR agonist
4-chloro-m-cresol, myotubes released IL-6; this was dependent
on de novo protein synthesis and was blocked by dantrolene
(a substance that specifically closes calcium channels, thereby
blocking calcium release from the SR) and cyclosporine (a
substance that blocks calcium-dependent calcineurin activation
by nuclear factor of activated T-cells) (
6). Moreover, in an
experiment in which caffeine was added to C2C12 skeletal
muscle cultured cells, Fang et al. observed that mM-level caffeine
secreted IL-6 in the culture supernatant (
17). However, this
report did not confirm whether caffeine administration affects
[Ca
2+
]i. In addition, it has not been confirmed whether the
caffeine-induced IL-6 secretion is suppressed by decreasing
[Ca
2+
]i. On the other hand, in an experiment in which IL-6 was
secreted by the contraction of C2C12 cultured skeletal muscle
cells induced by electrical stimulation, it was reported that the
increase in [Ca
2+
]i was more important than the contraction
of the cells themselves (
9). Therefore, an increase in [Ca
2+
]i
is considered to be important for the secretion of IL-6. These
results suggest that caffeine can regulate the secretion of IL-6
through an increase in [Ca
2+
]i. In addition, both physiologic al
concentrations and µM levels of caffeine also act directly on
skeletal muscle to bring about an ergogenic effect, and it is
thought that the mobilization of intracellular calcium is also
involved in this effect (
38). Conversely, caffeine did not affect
(i.e., did not induce) IL-6 vesicle secretion even after 70 min
of intravenous administration to mice at the highest possible
dose (85 mg/kg) (
11). In this study, confocal imaging was
used to visualize the endogenous IL-6 protein in glycolytic f ast
fibers of the tibialis anterior muscle of mice. Moreover, 2 h of
incubation of either the extensor digitorum longus (EDL) or
soleus muscle from mice with the Ca
2+
ionophore ionomycin
in the medium did not significantly increase IL-6 levels (39).
However, ionomycin stimulation showed a tendency of an
increase in IL-6 release from skeletal muscle, particularly from
soleus m uscle. On the other hand, caffeine induced the rele ase
of IL-6 from human myotubular cells, and its maximum release
occurred 4 to 6 h after the addition of caffeine (
6). Similarly,
incubation of isolated rat soleus muscle with ionomycin for
60 min in the incub ation media increased protein levels of IL-6
of (
40). A possible explanation of the discrepancy among these
previous studies regarding the secretion of IL-6 from skeletal
muscle could be owing t o differences in fiber type ( glycolytic vs.
oxidative) or animal species (rats vs. mice) used in each study.
Indeed, it has been known that rat soleus muscle contains more
oxidative fibers than mouse soleus muscle (
41). As IL-6 secretion
from the soleus muscle of rats is gre ater than that from the soleus
muscle of mice, differences in skeletal muscle fiber types in each
animal species may explain the differences in responsiveness of
IL-6 secretion to an increase in intracellular calcium by caffeine
and other Ca
2+
ionophores.
On the other hand, it has been reported that intravenous
acute AICAR stimulation (within 100 min) decreases the
number of IL-6-vesicles in mouse skeletal myocytes, suggesting
that AMPK activation can be involved in myokine secretion
(
11). In addition, incubation of cultured human myotubes
with AICAR within 4 to 24 h incre ases levels of IL-6 mRNA
(
42). These results suggest t hat AMPK signaling, one of the
mechanisms of the ergogenic effects of caffeine reported to date,
may regulate myokine expression and release.
Caeine could regulate the secretion of
brain-derived neurotrophic factor as a
myokine
Exercise incre ases circulating and muscle brain-derived
neurotrophic factor (BDNF), which is a myokine, in humans
and mice (
43). BDNF promotes f atty acid oxidation and
mitochondrial biogenesis in cultured skeletal muscle cells and
the skeletal muscle of mice (
43–45). We also found that
blood BDNF levels in healthy subjects and patients with heart
failure (HF) are closely positively correlated with whole-body
exercise capacity (peak oxygen uptake) by univariate analysis
and was identified as independent determinants of peak oxygen
uptake by multivariate analysis (
46). The administration of
recombinant human BDNF (rhBDNF), as well as exercise
training, improved whole-body exercise performance in normal
mice (
45).
BDNF has been shown to be secreted by the electrical
stimulation of skeletal muscle (
43). However, whether BDNF
secretion from skeletal muscle is re gulated by caffeine or
ionomycin remains unknown. The central effects of caffeine are
thought to depend on the release of various neurotransmitters
Frontiers in Sports and Active Living 03 frontiersin.org
Takada et al. 10.3389/fspor.2022.969623
by the inhibition of the adenosine receptors A1 and A2a (37).
Caffeine also has t he effect of reducing skeletal muscle pain
during exercise, which is thought to be associated with its
inhibition of adenosine receptor A1 (
37). Thus, the central
effects of caffeine and its effects on skeletal muscle are considered
to be similar. It is known that the addition of caffeine increases
BDNF secretion in cultured hippocampal neurons, which is due
to the increase in [Ca
2+
]i via the ryanodine receptor (47). It has
not yet been clarified whet her caffeine induces BDNF secretion
in skeletal muscle cells. However, given the similarities between
the central and peripheral effects of caffeine, BDNF could se crete
as a myokine via the caffeine-induced increase in [Ca
2+
]i.
Caeine could regulate the secretion of
musclin as a myokine
Musclin is expressed specifically in skeletal muscle (
29), and
is considered to be a myokine be cause exercise increases skeletal
muscle levels of musclin protein and mRNA, and circulating
levels of musclin (
8). The genetic disruption of musclin causes
a decrease in physical endurance and mitochondrial content,
including the signaling of mitochondrial biogenesis (
8). In
contrast, skeletal muscle-specific musclin overexpression using
adeno-associated virus 6 also increases circulating musclin (
29).
This suggests that skeletal muscle musclin can be secreted
into the circulation. On the other hand, mRNA expression
levels of mus clin have also been reported to increase in
a [Ca
2+
]i (addition of an ionophore and calcium itself)-
dependent manner in cultured murine and human primary
myoblasts (
8). Alt hough it has not yet been confirmed, caffeine
administration could have the potential to induce musclin
secretion by increasing the expression level of musclin.
Activation of AMPK regulates IL-15 as a
myokine
IL-15 is predominantly expressed in skeletal muscle (
12),
and is considered to be a myokine because exercise increases
skeletal muscle levels of both IL-15 protein and mRNA, and
circulating levels of the IL-15 protein (
12, 48). Similarly, AICAR,
an activator of AMPK, was found to increase skeletal muscle
IL-15 mR NA levels in mice (
12). Moreover, it is well known
that IL-15 promotes fatty acid and glucose oxidation, and
mitochondrial oxidative function with supercomplex formation
of the electron transport chain in muscle tissue (
49–53).
IL-15 also inhibit skeletal muscle degradation and muscle
nuclear apoptosis (
54–56), and increase muscle grip strength
(
12). Moreover, circulating IL-15 and skeletal mus cle IL-15Ra
expression correlated with protein synthesis after resistance
exercise (57). Furthermore, mice overexpressing IL-15 in skeletal
muscle on a low-fat/low-energy diet and a high-fat/high-energy
diet had increased lean body mass, including skeletal muscle
(58). As described above, IL-15 is considered to be a myokine
that has a generally positive effect on skelet al muscle, but
whether its expression and secretion can be regulated by caffeine
is a subject for future research.
Association of myokines with acute
and chronic eects of caeine
stimulation in vitro
The effects of exercise can be acute or chronic (1, 59).
Similarly, the effects of acute and chronic muscle contraction
are different (
10), but whether there is a difference in myokine
secretion is unclear. In this paper, we hypothesized and
discussed t hat the ergogenic effects of caffeine are mediated
by myokine secretion. Long-term (i.e., chronic) as well
as single, short-term (i.e., acute) administration of caffeine
produces ergogenic effects via intracellular calcium increases
and AMPK signaling (
Figure 1) (10). This is thought to
mimic the effects of acute and chronic exercise (
1, 59). From
the viewpoint of intracellular calcium increase and AMPK
signaling, myokine secretion is thought to play an important
role in the effects of caffeine. However, at present, IL-6 is
the only myokine that has been shown to be directly secreted
from skeletal muscle upon short-term caffeine s timulation
(
6). Therefore, comprehensive investigation of the types of
myokines that are secreted by caffeine stimulation is an
important research topic. In addition, research on myokine
secretion by chronic caffeine stimulation is also an unresolved
issue. It is well known that chronic caffeine administration
to skeletal muscle enhances glucose and lipid oxidation, and
mitochondrial biogenesis, which can be explained by the effects
of BDNF (
44, 45).
Indirect eects of myokines on other
organs
Caffeine intake markedly increases IL-6 levels in the skeletal
muscle and blood, but not in the liver of mice. Furthermore,
caffeine-stimulated skeletal muscle IL-6 production alleviated
nonalcoholic fatty liver disease (NAFLD) in a rodent model
(17). On the other hand, the overexpression of musclin
in skeletal muscle was found to attenuate left ventricular
dysfunction and myocardial fibrosis in mice with HF induced
by long-term pressure overload (
29). These results suggest
that caffeine ameliorates myocardial remodeling via inducing
crosstalk between the muscle and liver or heart. On the
other hand, mice overexpressing IL-15 in skeletal muscle
have reduced fat mass and show anti-obesity effects (
52, 58).
Frontiers in Sports and Active Living 04 frontiersin.org
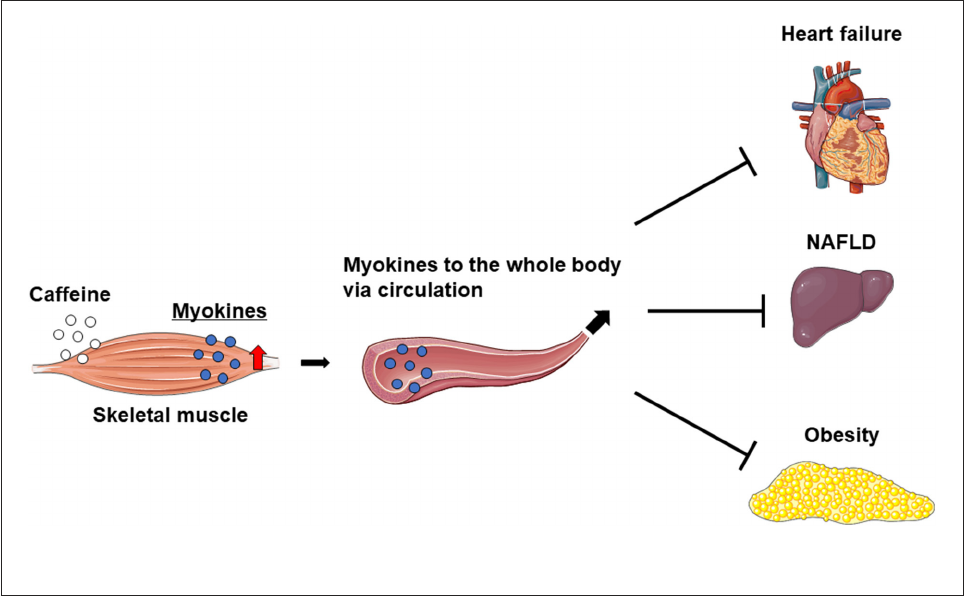
Takada et al. 10.3389/fspor.2022.969623
FIGURE 2
Increased myokines in skeletal muscle may improve heart failure, NAFLD, and obesity.
Therefore, increased myokine levels in the skeletal muscle
and circulation owing to exercise and/or the intake of
caffeine as an ergogenic aid may prevent or improve spe cific
pathologies, such as myocardial remodeling in HF, NAFLD, and
obesity (
Figure 2).
Future directions and perspectives
Why do the health benefits of exercise extend beyond
the skeletal muscles to the whole body? Although the
full mechanism still remains unclear (
1), the discovery of
molecules that are the key to the systemic effects of exercise,
namely myokines, has greatly advanced the field of exercise
physiology (
3, 28). Through the discovery of myokines,
which are specific molecules that have physiological activity
and can be secreted into the blood, it has been shown
that exercise and caffeine, an exercise mimetic, have effects
not only on skeletal muscle itself, but also on remote
organs via skeletal muscle (
4, 29, 60). In addition, many
mechanisms underlying the association between intracellular
calcium dynamics and intracellular transport and secretion
resulting from caffeine stimulation have been elucidated (
26,
27), and because intracellular calcium also demonstrates
characteristic dynamics during muscle contraction, it can be
speculated that myokine secretion into the blood is regulated by
exercise (
5, 28).
However, it should be reiterated that the intracellular events
reproduced by caffeine stimulation reflect some mechanisms
of some modes of exercise within the larger framework of
exercise. Indeed, it has been reported that the composition of
proteins in the blood changes with the intensity and type of
exercise performed (61), and hence attention should be paid
to what type of exercise is reproduced by caffeine. Caffeine
has the potential to increase our understanding of exercise-
induced myokine s ecretion and its systemic effects, which is
an interdisciplinary field between exercise physiology and cell
biology. Furthermore, clarifying the mechanism of myokine
secretion induced by exercise may help to resolve the effects
of physical inactivity in older people and enhance the efficacy
of post-injury rehabilitation. This is because some myokines
have already been found to be clinically significant (
46, 62, 63).
In addition, not surprisingly, patients facing clinical challenges
often have difficulty exercising on their own, so the systemic
effects of myokines induced by caffeine stimulation as an
exercise mimetic have great promise (
4, 64).
In this review, we conne cted “(1) the positive effects of
myokines on skeletal muscle” with “(2) the secretion of these
myokines by caffeine and their effects on skeletal muscle”
to form the hypotheses shown in Figure 1. The results of
(1) and (2) are from separate studies, and it hence remains
Frontiers in Sports and Active Living 05 frontiersin.org
Takada et al. 10.3389/fspor.2022.969623
unclear whether caffeine has acute or chronic effects on
skeletal muscle via myokines, and whether caffeine improves
exercise performance.
Conclusion
The ergogenic effects of caffeine are mediated thr ough
myokine regulation. Clarifying the underlying mechanisms will
require elucidation of not only the ergogenic effects of caffeine,
but also the mechanisms of the effects of exercise and myokines.
Author contributions
All authors listed have made a substantial, direct, and
intellectual contribution to the work and approved it for
publication.
Funding
This work was supported in part by Grants-in- Aid
for Scientific Research (Grant Nos. JP17H04758 to ST
and 21H03360 to SK) and Grants-in-Aid for Challenging
Exploratory Research (Grant No. 19K22791 to ST) from the
Japan Society for the Promotion of Science, grants from the
Akiyama Life Science Foundation (to ST), the Suhara Memorial
Foundation (to ST), and the Japan Foundation for Applied
Enzymology (to ST).
Acknowledgments
The authors thank H.A. Popiel for her critical reading of
the manuscript.
Conflict of interest
The authors declare that the research was conducted in the
absence of any commercial or financial relationships that could
be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the
authors and do not necessarily represent those of their affiliated
organizations, or those of t he publisher, the editors and the
reviewers. Any product that may be evaluated in this article, or
claim that may be made by its manufacturer, is not guaranteed
or endorsed by the publisher.
References
1. Egan B, Zierath JR. Exercise metabolism and the molecular
regulation of skeletal muscle adaptation. Cell Metab. (2013) 17:162–
84. doi: 10.1016/j.cmet.2012.12.012
2. Thyfault JP, Bergouignan A. Exercise and met abolic health: beyond skeletal
muscle. Diabetologia. (2020) 63:1464–74. doi: 10.1007/s00125-020-05177-6
3. Bay ML, Pedersen BK. Muscle-organ crosstalk: focus on immunometabolism.
Front Physiol. (2020) 11:567881. doi: 10.3389/fphys.2020.567881
4. Takada S, Sabe H, Kinugawa S. Abnormalities of skeletal muscle, adipocyte
tissue, and lipid metabolism in heart failure: practical therapeutic targets. Front
Cardiovasc Med. (2020) 7:79. doi: 10.3389/fcvm.2020.00079
5. Pedersen BK, Febbraio MA. Muscle as an endocrine organ:
focus on muscle-derived interleukin-6. Physiol Rev. (2008) 88:1379–
406. doi: 10.1152/physrev.90100.2007
6. Ducreux S, Zorzato F, Muller C, Sewry C, Muntoni F, Quinlivan R, et al. Effect
of ryanodine receptor mutations on interleukin-6 release and intracellular calcium
homeostasis in human myotubes from malignant hyperthermia-susceptible
individuals and patients affected by central core disease. J Biol Chem. (2004)
279:43838–46. doi: 10.1074/jbc.M403612200
7. Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin
(CTRP15), a novel myokine that links skeletal muscle to systemic lipid
homeostasis. J Biol Chem. (2012) 287:11968–80. doi: 10.1074/jbc.M111.
336834
8. Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SR, Reyes S, et al. Musclin is an
activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci
U S A. (2015) 112:16042–7. doi: 10.1073/pnas.1514250112
9. Furuichi Y, Manabe Y, Takagi M, Aoki M, Fujii NL. Evidence for acute
contraction-induced myokine se cretion by C2C12 myotubes. PLoS ONE. (2018)
13:e0206146. doi: 10.1371/journal.pone.0206146
10. Carter S, Solomon TPJ. In vitro experimental models for examining t he
skeletal muscle cell biology of exercise: the possibilities, challenges and future
developments. Pflugers Arch. (2019) 471:413–29. doi: 10.1007/s00424-018-2210-4
11. Lauritzen HP, Brandauer J, Schjerling P, Koh HJ, Treebak JT, Hirshman MF,
et al. Contraction and AICAR stimulate IL-6 vesicle depletion from skeletal muscle
fibers in v i vo. Diabetes. (2013) 62:3081–92. doi: 10.2337/db12-1261
12. Crane JD, Macneil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, et al. Exercise-
stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism
and aging. Aging Cell. (2015) 14:625–34. doi: 10.1111/acel.12341
13. Davis JK, Green JM. Caffeine and anaerobic performance:
ergogenic value and mechanisms of action. Sports Med. (2009)
39:813–32. doi: 10.2165/11317770-000000000-00000
14. Grgic J, Grgic I, Pickering C, Schoenfeld BJ, Bishop DJ, Pedisic Z. Wake
up and smell the coffee: caffeine supplementation and exercise performance-an
umbrella review of 21 published meta-analyses. Br J Sports Med. (2020) 54:681–
8. doi: 10.1136/bjsports-2018-100278
15. Martins GL, Guilherme J, Ferreira LHB, De Souza-Junior TP, Lancha AHJr.
Caffeine and exercise performance: possible directions for definitive findings. Front
Sports Act Living. (2020) 2:574854. doi: 10.3389/fspor.2020.574854
16. Kreutzer A, Graybeal AJ, Moss K, Braun-Trocchio R, Shah M. Caffeine
supplementation strategies among endurance athletes. Front Sports Act Living.
(2022) 4:821750. doi: 10.3389/fspor.2022.821750
17. Fang C, Cai X, Hayashi S, Hao S, Sakiyama H, Wang X, et al.
Caffeine-stimulated muscle IL-6 mediates alleviation of n on-alcoholic fatty
liver disease. Biochim Biophys Acta Mol Cell Biol Lipids. (2019) 1864:271–
80. doi: 10.1016/j.bbalip.2018.12.003
18. Mcconell GK, Ng GP, Phillips M, Ruan Z, Macaulay SL, Wadley
GD. Central role of nitric oxide synthase in AICAR and caffeine-induced
Frontiers in Sports and Active Living 06 frontiersin.org
Takada et al. 10.3389/fspor.2022.969623
mitochondrial biogenesis in L6 myocytes. J Appl Physiol. (2010) 108:589–
95. doi: 10.1152/japplphysiol.00377.2009
19. Lally JSV, Jain SS, Han XX, Snook LA, Glatz JFC, Luiken J, et al. Caffeine-
stimulated fatty acid oxidation is blunted in CD36 null mice. Acta Physiol. (2012)
205:71–81. doi: 10.1111/j.1748-1716.2012.02396.x
20. Moore TM, Mortensen XM, Ashby CK, Harris AM, Kump KJ, Laird DW,
et al. The effect of c affeine on skelet al muscle anabolic signaling and hypertrophy.
Appl Physiol Nutr Metab. (2017) 42:621–9. doi: 10.1139/apnm-2016-0547
21. Talanian JL, Spriet LL. Low and moderate doses of caffeine late in exercise
improve performance in trained cyclists. Appl Physiol Nutr Metab. (2016) 41:850–
5. doi: 10.1139/apnm-2016-0053
22. Ramirez-Maldonado M, Jurado-Fasoli L, Del Coso J, Ruiz JR, Amaro-
Gahete FJ. Caffeine increases maximal fat oxidation during a graded
exercise test: is there a diurnal variation? J Int Soc Sports Nutr. (2021)
18:5. doi: 10.1186/s12970-020-00400-6
23. Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev.
(1977) 57:71–108. doi: 10.1152/physrev.1977.57.1.71
24. Endo M. Calci um-induced calcium release in skeletal muscle. Physiol Re v.
(2009) 89:1153–76. doi: 10.1152/physrev.00040.2008
25. Maurya SK, Herrera JL, Sahoo SK, Reis FCG, Vega RB, Kelly DP, et al.
Sarcolipin signaling promotes mitochondrial biogenesis and oxidative metabolism
in skeletal muscle. Cell Rep. (2018) 24:2919–31. doi: 10.1016/j.celrep.2018.08.036
26. Olson EN, Williams RS. Calcineurin signaling and muscle remodeling. Cell.
(2000) 101:689–92. doi: 10.1016/S0092-8674(00)80880-6
27. Stojilkovic SS. Ca2+-regulated exocytosis and SNARE function. Trends
Endocrinol Metab. (2005) 16:81–3. doi: 10.1016/j.tem.2005.0 2.002
28. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a
secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
29. Szaroszyk M, Kattih B, Martin-Garrido A, Trogisch FA, Dittrich GM, Grund
A, et al. Skeletal muscle derived Musclin protects t he heart during pathological
overload. Nat Commun. (2022) 13:149. doi: 10.1038/s41467-021-27634-5
30. Egawa T, Hamada T, Ma X, Karaike K, Kameda N, Masuda
S, et al. Caffeine activates preferentially alpha1-isoform of 5’AMP-
activated protein kinase in rat skeletal muscle. Acta Physiol. (2011)
201:227–38. doi: 10.1111/j.1748-1716.2010.02169.x
31. Mathew TS, Ferris RK, Downs RM, Kinsey ST, Baumgarner BL. Caffeine
promotes autophagy in skeletal muscle cells by increasing the calcium-dependent
activation of AMP-activated protein kinase. Biochem Biophys Res Commun. (2014)
453:411–8. doi: 10.1016/j.bbrc.2014.09.094
32. Allen DL, Uyenishi JJ, Cleary AS, Mehan RS, Linds ay SF, Reed JM.
Calcineurin activates interleukin-6 transcription in mouse skeletal muscle i n vivo
and in C2C12 myotubes in vitro. Am J Physiol Regul Integr Comp Physiol. (2010)
298:R198–210. doi: 10.1152/ajpregu.00325.2009
33. Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio
MA, et al. Acute IL-6 treatment increases fatty acid turnover in elderly humans
in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab. (2005)
288:E155–162. doi: 10.1152/ajpendo.00257.2004
34. Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G,
et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and
glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase.
Diabetes. (2006) 55:2688–97. doi: 10.2337/db05-1404
35. Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K,
et al. Interleukin-6 directly increases glucose metabolism in resting human skeletal
muscle. Diabetes. (2007) 56:1630–7. doi: 10.2337/db06-1733
36. Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P.
Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle
hypertrophy. Cell Metab. (2008) 7:33–44. doi: 10.1016/j.cmet.2007.11.011
37. Mclellan TM, Caldwell JA, Lieberman HR. A review of caffeine’s effects on
cognitive, physical and occupational performance. Neurosci Biobehav Rev. (2016)
71:294–312. doi: 10.1016/j.neubiorev.2016.09.001
38. Tallis J, Duncan MJ, James RS. What can isolated skelet al muscle experiments
tell us about the effects of caffeine on exercise performance? Br J Pharmacol. (2015)
172:3703–13. doi: 10.1111/bph.13187
39. Glund S, Treebak JT, Long YC, Barres R, Viollet B, Wojtaszewski JF, et al.
Role of adenosine 5’-monophosphate-activated protein kinase in interleukin-6
release from isolated mouse skeletal muscle. Endocrinology. (2009) 150:600–
6. doi: 10.1210/en.2008-1204
40. Holmes AG, Watt MJ, Carey AL, Febbraio MA. Ionomycin,
but not physiologic doses of epinephrine, stimulates skeletal muscle
interleukin-6 mRNA expression and protein release. Metabolism. (2004)
53:1492–5. doi: 10.1016/j.metabol.2004.05.015
41. Gregorevic P, Meznarich NA, Blankinship MJ, Crawford RW, Chamberlain
JS. Fluorophore-labeled myosin-specific antibodies simplify muscle-fiber
phenotyping. Muscle Nerve. (2008) 37:104–6. doi: 10.1002/mus.20877
42. Weigert C, Dufer M, Simon P, Debre E, Runge H, Brodbeck K, et al.
Upregulation of IL-6 mRNA by IL-6 in skeletal muscle cells: role of IL-6 mRNA
stabilization and Ca2+-dependent mechanisms. Am J Physiol Cell Physiol. (2007)
293:C1139–1147. doi: 10.1152/ajpcell.00142.2007
43. Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O,
et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in
response to contraction and enhances fat oxidation via activation of AMP-activated
protein kinase. Diabetologia. (2009) 52:1409–18. doi: 10.1007/s00125-009-1364-1
44. Matsumoto J, Takada S, Kinugawa S, Furihata T, Nambu H,
Kakutani N, et al. Brain-derived neurotrophic factor improves limited
exercise capacity in mice with heart failure. Circulation. (2018)
138:2064–6. doi: 10.1161/CIRCULATIONAHA.118.035212
45. Matsumoto J, Takada S, Furihata T, Nambu H, Kakutani N, Maekawa
S, et al. Brain-Derived neurotrophic factor improves impaired fatty
acid oxidation via the activation of adenosine monophosphate-activated
protein kinase-a - proliferator-activated receptor-r coactivator-1a sign aling
in skeletal muscle of mice with heart failure. Circ Heart Fail. (2021)
14:e005890. doi: 10.1161/CIRCHEARTFAILURE.119.005890
46. Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Yokota T,
et al. Decreased serum brain-derived neurotrophic factor levels are correlated
with exercise intolerance in patients with heart failure. Int J Cardiol. (2013)
168:e142–144. doi: 10.1016/j.ijcard.2013.08.073
47. Lao-Peregrin C, Ballesteros JJ, Fernandez M, Zamora-Moratalla A, Saavedra
A, Gomez Lazaro M, et al. Caffeine-mediated BD NF release regulates long-
term synaptic plasticity through activation of IRS2 signaling. Addict Biol. (2017)
22:1706–18. doi: 10.1111/adb.12433
48. Yang H, Chang J, Chen W, Zhao L, Qu B, Tang C, et al. Treadmill exercise
promotes interleukin 15 expression in skeletal muscle and interleukin 15 receptor
alpha expression in adipose tissue of high-fat diet rats. Endocr i ne. (2013) 43:579–
85. doi: 10.1007/s12020-012-9809-6
49. Almendro V, Busquets S, Ametller E, Carbo N, Figueras M, Fuster G,
et al. Effects of interleukin-15 on lipid oxidation: disposal of an oral [(14)C]-
triolein load. Biochim Biophys Acta. (2006) 1761:37–42. doi: 10.1016/j.bbalip.2005.
12.006
50. Busquets S, Figueras M, Almendro V, Lopez-Soriano FJ, Argiles
JM. Interleukin-15 increases glucose uptake in skeletal muscle. An
antidiabetogenic effect of the cytokine. Biochim Biophys Acta. (2006)
1760:1613–7. doi: 10.1016/j.bbagen.2006.09.001
51. Krolopp JE, Thornton SM, Abbott MJ. IL-15 Activates the Jak3/STAT3
signaling pathway to mediate glucose uptake in skeletal muscle cells. Front Physiol.
(2016) 7:626. doi: 10.3389/fphys.2016.00626
52. Nadeau L, Aguer C. Interleukin-15 as a myokine: mechanistic insight into
its effect on skeletal muscle metabolism. Appl Physiol Nutr Metab. (2019) 44:229–
38. doi: 10.1139/apnm-2018-0022
53. Nadeau L, Patten DA, Caron A, Garneau L, Pinault-Masson E, Foretz M,
et al. IL-15 improves skeletal muscle oxidative metabolism and glucose uptake
in association with increased respiratory chain supercomplex formation and
AMPK pathway activation. Biochim Biophys Acta Gen Subj. (2019) 1863:395–
407. doi: 10.1016/j.bbagen.2018.10.021
54. Carb o N, Lopez-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM,
et al. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J
Cancer. (2000) 83:526–31. doi: 10.1054/bjoc.2000.1299
55. Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM.
Overexpression of interleukin-15 induces skeletal muscle hypertrophy i n vitro:
implications for treatment of muscle wasting disorders. Exp Cell Res. (2002)
280:55–63. doi: 10.1006/excr.2002.5624
56. Busquets S, Figueras MT, Meijsing S, Carbo N, Quinn LS, Almendro V, et al.
Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol
Med. (2005) 16:471–6. doi: 10.3892/ijmm.16.3.471
57. Perez-Lopez A, Mckendry J, Martin-Rincon M, Morales-Alamo D, Perez-
Kohler B, Valades D, et al. Skeletal muscle IL-15/IL-15Ralpha and myofibrillar
protein synthesis after resistance exercise. Scand J M ed Sci Sports. (2018) 28:116–
25. doi: 10.1111/sms.12901
58. Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM.
Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am
J Physiol Endocrinol Metab. (2009) 296:E191–202. doi: 10.1152/ajpendo.90506.
2008
59. Chow LS, Gerszten RE, Taylor JM, Pedersen BK, Van Praag
H, Trappe S, et al. Exerkines in health, resilience and disease.
Frontiers in Sports and Active Living 07 frontiersin.org
Takada et al. 10.3389/fspor.2022.969623
Nat Rev Endocrinol. (2022) 18:273–89. doi: 10.1038/s41574-022-
00641-2
60. Gubert C, Hannan AJ. Exercise mimetics: harnessing the
therapeutic effects of physical activity. Nat Rev Drug Discov. (2021)
20:862–79. doi: 10.1038/s41573-021-00217-1
61. Morville T, Sahl RE, Moritz T, Helge JW, Clemmensen C. Plasma metabolome
profiling of resistance exercise and endurance exercise in humans. Cell Rep. (2020)
33:108554. doi: 10.1016/j.celrep.2020.108554
62. Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Yokota
T, et al. Serum brain-derived neurotropic factor level predicts adverse
clinical outcomes i n patients with heart failure. J Card Fail. (2015) 21:300–
6. doi: 10.1016/j.cardfail.2015.01.003
63. Nakano I, Kinugawa S, Hori H, Fukushima A, Yokota T, Takada S, et al.
Serum brain-derived neurotrophic factor levels are associated with skeletal muscle
function but not wi th muscle mass in patients with heart failure. Int Heart J. (2020)
61:96–102. doi: 10.1536/ihj.19-400
64. Takada S, Sabe H, Kinugawa S. Treatments for skeletal muscle abnormalities
in heart failure: sodium-glucose transporter 2 and ketone bodies. Am J
Physiol Heart Circ Physiol. (2022) 322:H117–28. doi: 10.1152/ajpheart.00100.
2021
Frontiers in Sports and Active Living 08 frontiersin.org
